Share price
100
Million patients in the EU and the US has nail fungus
76
%
Of the patients become fungus-free in phase 3 for MOB-015
13
MOB-015 has market approval in 13 EU countries
1
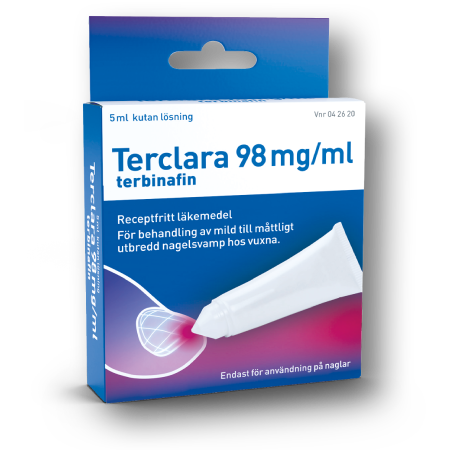
Terclara® is the market leader in Sweden and Norway
Latest Report
Next Event

Our business model means lower development risk and greater opportunities to reach the market quickly than traditional drug development.